Interview with Roberto Magnatantini, Senior Equity Fund Manager and Iana Perova, Equity Analyst
- Medical Revolution Underway: Discover how precision medicine is transforming standard treatments into tailor-made therapies based on a patient’s genetics.
- Personalized Outcomes: Dive into a world where individual healthcare outcomes surpass the traditional one-size-fits-all approach.
- Addressing Societal Challenges: Understand how this breakthrough can alleviate issues posed by an aging population, offering sustainable solutions.
- Promising Investment Opportunities: Explore the long-term investment potential presented by the booming sector of precision medicine.
Earlier this year, we introduced our new Wellness Series, where we shine a spotlight on the most captivating health trends, bringing you insights into the most recent and intriguing developments in the realm of well-being.
What used to be the stuff of futuristic novels is now starting to become reality: medical treatments that are tailored to a patient’s physiological characteristics (genetic notably), rather than being standard “one-size fits all” prescriptions. This not only yields better outcomes at the individual level, but also has the potential to help address some of the societal challenges posed by an ageing population. Making for promising long-term investment opportunities too…
Iana and Roberto, could you start by explaining what is meant by precision medicine, and how it differs from the traditional approach still largely used by physicians across the globe?
Old school medicine, as it is indeed still largely practised, comes into play only once a health issue arises. It then works on the basis of averages: a patient’s symptoms are ascribed to the condition that usually produces them, and this diagnosis then leads to a treatment using the drugs that work best for the average person. If such treatment fails to deploy positive effects, a different drug will be prescribed, or the initial diagnosis might be questioned – in what is very much a “trial-and-error” process.
By contrast, precision medicine, also called personalised or individualised medicine, can intervene even before a patient feels ill, enables an earlier and more accurate diagnosis of the condition and recognises from the onset that one size does not fit all when it comes to treating that condition. Drugs that yield good results for some patients can be totally ineffective – or even cause adverse side-effects – on others.
A better understanding of this human heterogeneity enables physicians to deploy a much more targeted approach, whereby a patient receives the treatment that is best suited to his or her condition. In certain cases, medical interventions are even used to modify the patient’s genetic makeup , in order to make the treatment more effective.
Percentage of the patient population for which a particular drug in a class is ineffective, on average.
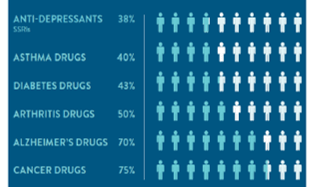
Reproduced with permission from: Spear,BB, Health-Chiazzi, M, Huff, J. Clinical application of pharmacogenetics. Trneds in Molecular Medicine, 2001;7(5):201-204.
Source: Raymond James Research
What is driving the development of such precision medicine, placing the focus on the individual – rather than average – patient?
There is no question that the significant advances in genomic research during the past decade, notably in terms of gene sequencing technologies and genomic profiling, sparked the advent of precision medicine. The massive drop in the cost of sequencing – and speed-up of the procedure – are adding further fuel. For while a patient’s environment and lifestyle are part of what explains his/her response to a treatment, individual genetic specificities will play a role that can be determinant.
In the life sciences sector, prominent players such as Thermo Fisher Scientific, or more specialised companies like Tecan, are key providers of a diverse array of gene sequencing solutions. These solutions encompass everything from library preparation kits to sequencing reagents and bioinformatics software. By providing comprehensive offerings, they simplify the entire sequencing process, thereby enhancing accessibility and efficiency for researchers.
Let us now talk about the first stage of precision medicine: detection. In what way do precision medicine tools help detect and precisely diagnose a patient’s condition? And how big is the opportunity for companies active in this field?
The opportunity is considerable ! According to a Fortune Business Insights report, the global precision diagnostics market stands to more than double between 2021 (USD 60 billion) and 2028 (USD 139 billion). The availability of new tools, notably whole genome sequencing (i.e. the determination at a single moment of the full DNA sequence of an organism’s genome), will be a key driver of this growth. But other factors are also at play: pressure to reduce healthcare costs by receiving diagnoses as early as possible, limiting the number of misdiagnoses, and improving patient information.
From a disease category standpoint, much of the growth – in the near-term at least – will pertain to oncology. Indeed, cancer currently figures among the main causes of death and the World Health Organisation estimates that the number of new cases will increase from 20 million worldwide in 2022 to 32.6 million in 2045 – in sync with ageing demographics. In cancer diagnosis, precision medicine tools enable analysis of the genetic mutations in a patient’s tumour cells, crucial for the selection of targeted therapies likely to yield the best results. An example being Roche’s FoundationOne assay, a blood test that identifies genomic alterations in more than 300 cancer-related genes.
In essence, by analysing molecular markers/genetic mutations, clinicians should be increasingly able to accurately diagnose diseases, predict their progression, and determine the most effective treatment strategies tailored to individual patients. Thus potentially saving both money and many lives.
Exciting prospects indeed! And what about the therapeutic stage, following the delivery of an accurate diagnosis? Please shed some light on how precision medicine is revolutionising practices in this field too.
As mentioned at the onset of this interview, once their patient’s condition has been assessed, doctors generally start by prescribing the standard first-line therapy available for that condition.
Despite the fact that such therapy is known to be ineffective for many, if not a majority, of patients. Indeed, studies put the ratio of non-effectiveness at 38% for anti-depressants, 50% for arthritis drugs and even 75% for cancer drugs.
When practising “new school” precision medicine, physicians will be able to rely on pharmacogenomic tests, that analyse a person’s genetic makeup to predict how they will respond to certain medications, in order to immediately prescribe the drug most suited to their patient – and generally also with lesser side effects. Precious time should thus be gained.
Success probability for new drugs in the U.S. with or w/o selection biomarkers by development phase between 2011 and 2020
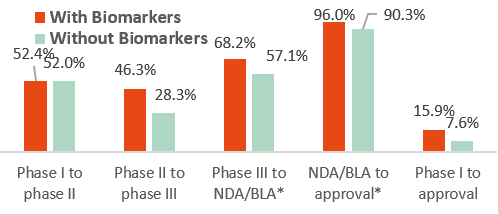
NDA= New Drug Applications, BLA= Biologics License Applications
Source: Statista
One step further lies cell therapy, whereby the genetics of a patient’s (or a donor’s) cells are modified to target specific tumour antigens (aka markers). This is already reality for some forms of blood cancers, and the field of applications is set to expand even to areas outside of oncology, such as autoimmune diseases and fibrosis. Interestingly, studies are also underway to determine how mRNA vaccines (turned famous by the Covid pandemic!) can be used to deliver cell therapies.
Not to forget the big potential of gene therapy, as a manner to treat – perhaps even cure – a number of hereditary diseases that stem from single gene mutations. As of today, only a few such therapies have been approved, pertaining to extremely rare diseases.
But here too lies the promise of treatment for conditions, like haemophilia A, that are more broadly prevalent.
The gene editing therapies co-developed by Vertex and CRISPR use the CRISPR-Cas9 technology to modify DNA within the human body, targeting the root cause of diseases at the molecular level. This revolutionary approach allows for the correction of genetic mutations that cause diseases, offering the possibility of permanent cures rather than merely treating symptoms. Their collaboration has already led to promising treatments for sickle cell disease and beta thalassemia, two genetically inherited blood disorders.
How is patient follow-up performed in the precision medicine model?
Once patients begin receiving a treatment, they undergo regular monitoring using tools such as blood tests, imaging and wearables to track their physiological response and detect any adverse effects early. The data is analysed to evaluate treatment effectiveness and safety. Based on this analysis and patient feedback, healthcare providers adjust treatment plans, potentially modifying dosages or medications to optimise outcomes and minimise side effects. Minimal Residual Disease (MRD) blood-based tests, for instance, which detect the presence of small numbers of cancer cells in the blood after treatment, are increasingly used to assess treatment effectiveness, predict relapse risk, confirm remission, and monitor for cancer recurrence.
All this indeed sounds very promising from a science perspective. But broad-based adoption of precision medicine will require – like all paradigm shifts – some catalysts. What could these be?
You are correct. How fast precision medicine takes off will depend on the incentives for adoption, at both the provider and the patient level. As regards providers, most continue for now to be reimbursed in terms of products and services delivered. Few are the countries that have moved to value-based healthcare, which clearly plays in favour of precision medicine.
Indeed, when providers are rewarded on the basis of patient outcome, they have an incentive to use all available tools and technologies such that the most effective treatment be prescribed – and fast.
Patients too need to be – financially – convinced of the benefits of better caring for one’s health, as the key to enhanced ageing. This means not only engaging in preventive activities, but also accepting to undergo regular screening and wear monitoring devices. Not to mention being willing for such data to be shared across the healthcare chain and being open to new forms of therapy. There is clearly an affordability issue here (i.e. the need to adapt public and private health insurance systems), but also one of mentality. That said, once a patient recognises that a personalised treatment works better and/or involves less side-effects, adherence to the treatment may well increase. And thus set a virtuous cycle into motion…
Speaking of patient data, right now AI is all the rage on financial markets. What role do data analytics in general, and AI in particular, stand to play in a world of precision medicine?
The three defining properties of “big data” are volume, variety and velocity – otherwise known as the 3 Vs. This applies particularly well to healthcare data, with fast accumulating quantities of sometimes very complex patient data. By this we mean healthcare records of course, but also real-time information coming from wearable devices.
Full deployment of precision medicine requires that all this data flows seamlessly and that computational tools enable its analysis. In this respect, AI will definitely play a key role.
A company such as GlobalData, for instance, provides vast datasets that cover data on clinical trials, including trial protocols, patient demographics, and treatment outcomes. Such data helps researchers identify relevant clinical trials for specific patient populations and optimise trial design for precision medicine interventions.
You have convincingly described the promises of precision medicine, as well as catalysts for adoption. There are no doubt a number of headwinds though, which our readers should also be aware of?
As with all paradigm shifts, there will certainly be headwinds to overcome. In the case of precision medicine, we should first mention a point that we alluded to earlier: patient acceptance. Convincing patients to share their private data and to accept novel treatment methods could indeed prove a challenge.
Another potential roadblock is the need for pharmaceutical and biotech companies to design smarter/more complex clinical trials, with a narrower target population. Indeed, whereby clinical trials have traditionally been designed to determine “the best outcome for the most” approach, smart clinical trials for targeted therapies are now transforming medical research. Pharmaceutical companies are increasingly tending to outsource trial management to specialised Clinical Research Organizations (CROs) such as IQVIA and ICON plc, which boast expertise in patient recruitment and trial coordination, essential for streamlined and effective trials.
Central to securing regulatory approval is demonstrating a treatment’s efficacy. This necessitates precise trial design encompassing patient selection, dosage determination and treatment duration. The complexity of targeted therapies further underscores the importance of smart trial planning, particularly in identifying and categorising patients based on biomarkers or genetics. Alongside the treatment’s active ingredient, meticulous trial design is thus essential for both treatment success and regulatory approval.
In healthcare, coverage and reimbursement for high-cost precision therapies also pose challenges. Insurers demand evidence of effectiveness, complicating matters when patients lack coverage. Collaboration between therapy developers and payers for outcome-centered payment models may drive precision medicine adoption amidst a shift to value-based healthcare.
On a final note, could you touch on the all-important subject of prevention and how precision medicine might revolutionise that too?
Prevention is pivotal in reshaping healthcare, exemplified by the case of type 2 diabetes. It stands as the costliest chronic disease in the US, incurring $237 billion in direct expenses and $90 billion in lost productivity yearly. Studies show lifestyle changes and early metformin use (the standard first line treatment) can slash its incidence by 58% and 31%*, respectively. Fast-forward to the current era of precision medicine. By better understanding who is at risk of becoming diabetic (i.e. assessing genetic predisposition to the disease) and thus delivering prophylactic therapy, it can help prevent the condition from actually taking hold, in contrast to reactive treatments post-diagnosis. Precision diagnostics further amplify prevention efforts, enabling early disease detection, monitoring, and personalized treatments, opening avenues for innovative diagnostic tools.
* Diabetes Prevention Program Research Group, N Engl J Med 2002

Roberto Magnatantini, CFA, Senior Portfolio Manager
Iana Perova, Equity Analyst
About DECALIA’s strategies exposed to the subject of this newsletter:
- DECALIA Silver Generation is a thematic strategy investing into companies that will structurally benefit from the longevity trend. The strategy intends to capture opportunities across the full spectrum of the longevity value chain: Consumption plays, Healthspan plays and Transformational companies.
- DECALIA Sustainable SOCIETY is a multi-thematic strategy investing in the 7 themes (Security, O2 & Ecology, Cloud & Digitalisation, Industrial 5.0, Elder & Well-being, Tech Med, Young Generation) that will shape tomorrow’s SOCIETY. The Elder & Well-being & Tech Med themes currently represent 22% of the fund.
- Both strategies are managed by an experienced investment team.
About DECALIA SA
Established in 2014, DECALIA SA is a Swiss investment management company. With more than 70 employees and assets under management that stand at €4.9 billion, DECALIA has expanded rapidly, in particular thanks to its active-management experience built up over the last 30 years by its founders. The strategies developed by DECALIA focus on four investment themes deemed promising in the long term: the disintermediation of the banking sector, the search for yield, long-term trends and market inefficiencies. DECALIA is regulated by FINMA through a collective assets manager’s license. In addition to its Geneva headquarter, the group has offices in Zurich, Milan & distributors of the DECALIA Sicav in Spain & Germany.
